Highlights:
- Glenmark’s FabiFlu gets approved in India to treat COVID-19 patients
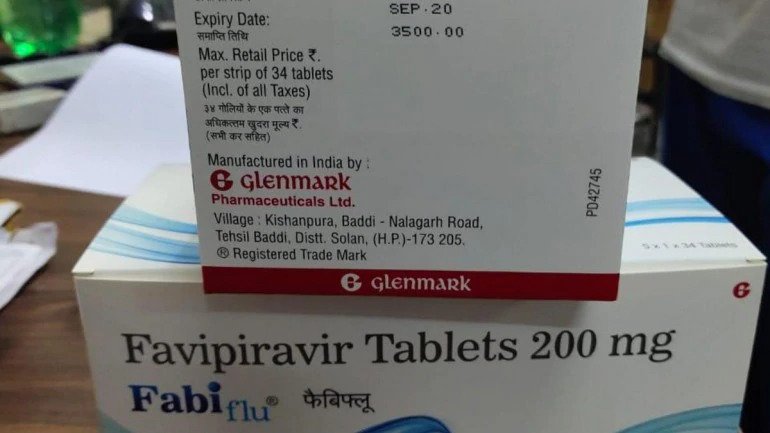
- One tablet of FabiFlu will cost Rs. 103
- A pack of 34 tablets of FabiFlu will cost Rs. 3,500
Glenmark Pharmaceuticals has launched a new antiviral drug named Favipiravir with brand name FabiFlu which will be used to treat mild to moderate cases of Coronavirus in India. The Mumbai based pharma company has become the 1st company in India to commercially launch an antiviral for the novel Coronavirus.
The Indian drug regulator, Drug Controller General of India (DCGI) has given Glenmark the green flag to market and manufacture the antiviral which was launched in India today.
The price of FabiFlu is kept at Rs. 103 per tablet which means a pack of 34 tablets will cost Rs. 3,500. The dosage if 200 MG X 9 tablets on the 1st days and 200 MG X 4 tablets a day for the 14 days of the treatment. However, the company clearly specifies that the tablet should only be taken if a doctor prescribes it.
In the clinical trials, the company conducted on 90 mild and 60 moderate patients of the novel Coronavirus in India across 11 locations in India, this drug claimed to have an efficiency of more than 80% in the treatment of these patients.
As per reports, there are many other pharma firms in India which have applied for approvals and are readying themselves to launch their drugs in India, including, Bengaluru-based Strides Pharma, Delhi-based Brinton Pharmaceuticals, Mumbai-based Lasa Supergenerics and Hyderabad-based Optimus Pharma.
Also Read: “Bat Woman” Of China Warns Coronavirus Only Scratch On The Surface
The Active Pharmaceutical Ingredient (API) and the formulation for FabiFlu were developed by Glenmark Pharmaceuticals via in-house Research and Development (R&D). The DCGI allowed fast track trials of the drug with Phase III in limited patients. The approval process is also under Emergency Use Authorisation (EUA) as of now.
With more than 13,000 deaths and a fatality rate of over 3% in India, the country is looking at 4,12,210 cases of the novel Coronavirus.
Also Read: What Are The Impact of COVID-19 On Education In India?
Glenn Saldanha, Chairman and Managing Director of Gelnmark Pharmaceuuticals said, “The approval comes at a time when cases in India are spiralling like never before and putting tremendous pressure on our healthcare system. FabiFlu will reduce this pressure. Glenmark will work with the government and medical community to make it quickly accessible to patients across the country,”.
The company, reportedly said, this drug acts by getting into the cells and then inhibits the activity of the viral replication to reduce the viral load. Early use of FabiFlu can control the huge rate of viral replication. However, if it is not controlled at an early stage, the viral replication slows down in the later stages which only results in complications due to the violent immune response by the human body which could lead to organ failure.
Having said this, the healthcare experts say this is not a “magic drug”, but can help amidst the surging cases in the country. Dr Vikar Maurya who is the Director of Department of Pulmonology and Sleep Disorders at Fortis Hospital, Shalimar Bagh, said, “It is not a magic bullet as it is not the only thing we have to give. This is not a specific drug made for Covid-19 and has been found to be useful, but how much it will be useful we will have to see. Real efficacy will be known when administered on a large scale,”.
Dr Maurya added, “The best thing is that it is an oral drug, while Ramdesiver is an intravenous drug. It (Favipiravir) can be even taken at home. So even if it is giving some benefit, it will be quite useful”.
Glenmark is also undertaking a study on COVID-19 patients where it is combining two antiviral drugs, Favipiravir (the drug approved for novel flu pandemics) and Umifenovir (drug approved for Influenza).
As of now, Favipiravir is already being used commercially in the Therapeutic Management of COVID-19 in both Bangladesh and UAE, it is sold under the brand name Avigan by Fujifilm Toyama Chemical and was already approved in Japan since 2014 as a viable treatment for Influenza.
Also Read: Donald Trump Claims Both India And China Will have More COVID-19 Patients If Testing Is Increased
A report in a leading news agency in India said, “In Japan, the drug has been approved for compassionate use on 2,050 Covid-19 patients. It’s also approved for novel or re-emerging pandemic influenza virus infection in the country. A trial of 760 patients of Favipiravir is underway in Canada. A couple of studies in China had also shown promising results. A Russian study among 390 patients had shown 80 per cent plus success rate and a trial among 2,141 patients in Japan showed above 88 per cent success rate.”
The Associate Director of Internal Medicine at Max Healthcare, Dr Rommel Tickoo said that drug is a “potential game-changer”.
He said, “We don’t have much data, but whatever data we have shows that it is promising. We will have clearer information about the efficacy of the drug in the next two months. The preliminary report is promising which means that they (Glenmark Pharmaceuticals) know that it works,”.
Dr Tickoo explains, “It has to be given at an early stage and is a potential game-changer as it can be given in tablet form and thus is easy to administer, and is relatively inexpensive,”.
Delhi’s Sir Ganga Ram Hospital’s Dr. Arvind Kumar who is a noted lung surgeon said that he does not believe that any of these antivirals drugs such as Favipiravir or Remdisiver will be game changer.
He said, “If at all ‘game changer’ can be used, it is for dexamethasone which has shown a significant reduction in mortality and is available cheaply,”.